Class 12 Chemistry
Chapter 12 – Aldehydes, Ketones and Carboxylic Acids
Chapter Notes
Overview of Aldehydes & Ketones
These are the organic compounds containing carbon-oxygen double bond (>C=O) i.e., carbonyl group.

When carbon of acyl group is attached to nitrogen it is called an amide.
When carbon of acyl group is attached to halogen it is called an acyl halide.
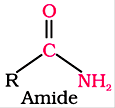

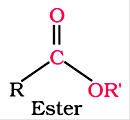
Derivatives of carboxylic acids consist of esters and anhydrides.

Structure of the Carbonyl Group
The carbonyl carbon atom is sp2-hybridised and forms three sigma (σ) bonds. The fourth valence electron of carbon remains in its p-orbital and forms a π-bond with oxygen by overlap with p-orbital of an oxygen.
The carbonyl carbon and the three atoms attached to it lie in the same plane and the π-electron cloud is above and below this plane.
The bond angles are approximately 120° as expected of a trigonal coplanar structure.

The carbon-oxygen double bond is polarised due to higher electronegativity of oxygen relative to carbon. Hence, the carbonyl carbon is an electrophilic (Lewis acid), and carbonyl oxygen, a nucleophilic (Lewis base) centre.
Carbonyl compounds have substantial dipole moments and are polar than ethers. The high polarity of the carbonyl group is explained on the basis of resonance involving a neutral (A) and a dipolar (B) structures as shown.

Nomenclature of Aldehydes and ketones
(a) Common names
The common names of most aldehydes are derived from the common names of the corresponding carboxylic acids, by replacing the ending –ic of acid with aldehyde.
The location of the substituent in the carbon chain is indicated by Greek letters α, β, γ, δ, etc.
The α-carbon being the one directly linked to the aldehyde group, β-carbon the next, and so on.

The common names of ketones are derived by naming two alkyl or aryl groups bonded to the carbonyl group.
The locations of substituents are indicated by Greek letters, α α′, β β′ and so on beginning with the carbon atoms next to the carbonyl group, indicated as αα′.
Some ketones have historical common names, the simplest dimethyl ketone is called acetone.
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.

(b) IUPAC names
The IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending –e with –al and –one respectively.
In case of aldehydes the longest carbon chain is numbered starting from the carbon of the aldehyde group.
In case of ketones the numbering begins from the end nearer to the carbonyl group.
In cyclic ketones, carbonyl carbon is numbered one.
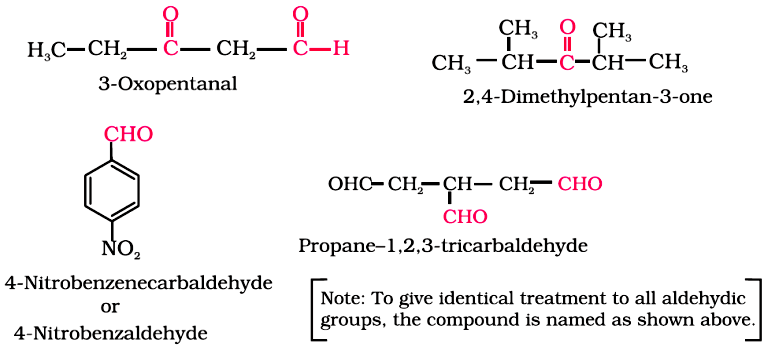
When the aldehyde group is attached to a ring, the suffix carbaldehyde is added after the full name of the cycloalkane. In this case, the numbering of the ring carbon atoms starts from the carbon atom attached to the aldehyde group.
The name of the simplest aromatic aldehyde carrying the aldehyde group on a benzene ring is benzenecarbaldehyde. However, the common name benzaldehyde is also accepted by IUPAC. Other aromatic aldehydes are hence named as substituted benzaldehydes.

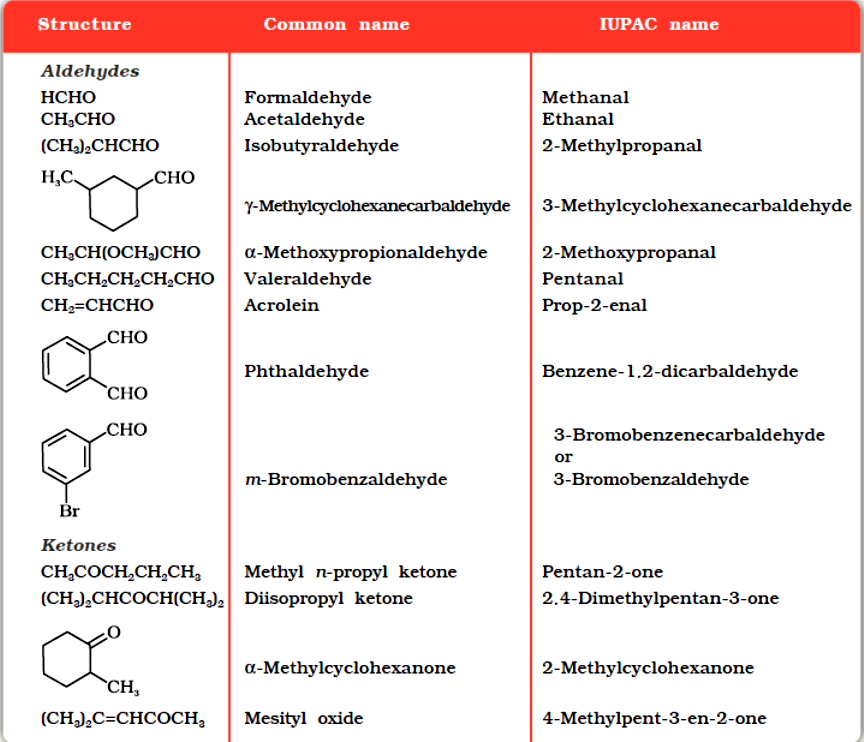
Common and IUPAC Names of Some Aldehydes and Ketones
1. From acyl chloride (acid chloride)
2. From nitriles and esters
3. From hydrocarbons
(i) By oxidation of methylbenzene
(ii) By side chain chlorination followed by hydrolysis
(iii) By Gattermann – Koch reaction
1. From acyl chloride (acid chloride)

(i) By Stephen reaction of nitriles
![]()
(ii) By selective reduction of nitriles
Nitriles are selectively reduced by diisobutylaluminium hydride, (DIBAL-H) to imines followed by hydrolysis to aldehydes.

(iii) By reduction of esters to aldehydes with DIBAL-H.

(i) By oxidation of methylbenzene
(a) By Etard reaction
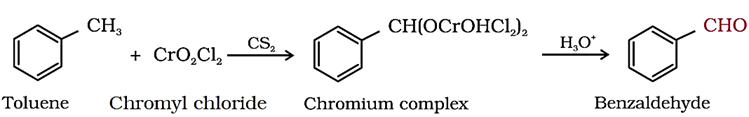
(b) From Toluene or Substituted Toluene
Toluene or substituted toluene is converted to benzylidene diacetate on treating with chromic oxide (CrO3) in acetic anhydride. The benzylidene diacetate can be hydrolysed to corresponding benzaldehyde with aqueous acid.

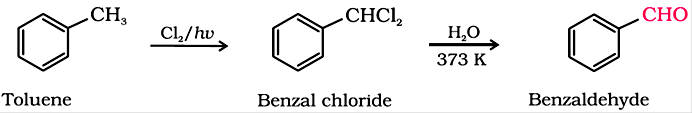
(ii) By side chain chlorination followed by hydrolysis
(commercial method of manufacture of benzaldehyde)
(iii) By Gattermann – Koch reaction
When benzene or its derivative is treated with carbon monoxide and hydrogen chloride in the presence of anhydrous aluminium chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.

1. From acyl chlorides
2. From nitriles
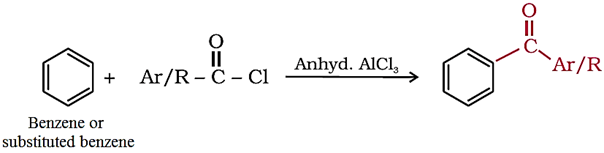
3. From benzene or substituted benzenes
Treatment of acyl chlorides with dialkylcadmium (R2Cd), prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.

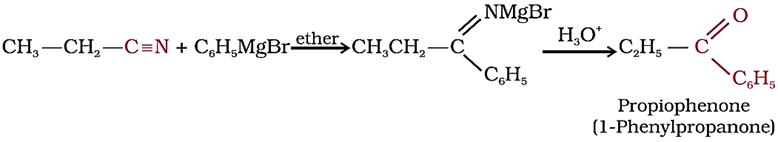
3. From benzene or substituted benzenes (Friedel-Crafts acylation)
Physical Properties of Aldehydes & Ketones
Methanal is a gas at room temperature. Ethanal is a volatile liquid.
Other aldehydes and ketones are liquid or solid at room temperature.
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses. It is due to weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
Their boiling points are lower than those of alcohols of similar molecular masses due to absence of intermolecular hydrogen bonding.
The following compounds of molecular masses 58 and 60 are ranked in order of increasing boiling points.
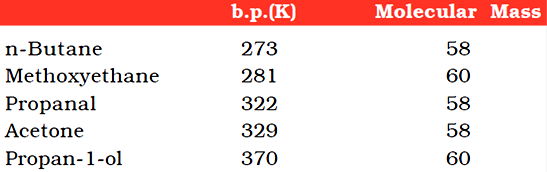
The lower members of aldehydes and ketones such as methanal, ethanal and propanone are miscible with water in all proportions, because they form hydrogen bond with water.
The solubility of aldehydes and ketones decreases rapidly on increasing the length of alkyl chain.
All aldehydes and ketones are fairly soluble in organic solvents like benzene, ether, methanol, chloroform, etc.
The lower aldehydes have sharp pungent odours. As the size of the molecule increases, the odour becomes less pungent and more fragrant. In fact, many naturally occurring aldehydes and ketones are used in the blending of perfumes and flavouring agents.
Chemical Reactions of Aldehydes and Ketones
1. Nucleophilic addition reactions
2. Reduction
(i) Reduction to alcohols
(ii) Reduction to hydrocarbons
3. Oxidation
(i) Tollens’ test
(ii) Fehling’s test
(iii) Oxidation of methyl ketones by haloform reaction
4. Reactions due to α-hydrogen
(i) Aldol condensation
(ii) Cross aldol condensation
5. Cannizzaro reaction
6. Electrophilic substitution reaction
1. Nucleophilic addition reactions
Aldehydes and ketones undergo nucleophilic addition reactions.
Mechanism:

The hybridisation of carbon changes from sp2 to sp3.
Reactivity:
Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons.
Sterically, the presence of two relatively large substituents in ketones hinders the approach of nucleophile to carbonyl carbon than in aldehydes having only one such substituent.
Electronically, aldehydes are more reactive than ketones because two alkyl groups reduce the electrophilicity of the carbonyl more effectively than in former.
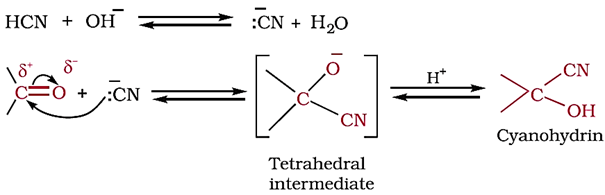
Nucleophilic addition of hydrogen cyanide (HCN):
Aldehydes and ketones react with hydrogen cyanide (HCN) to yield cyanohydrins.
This reaction occurs very slowly with pure HCN. Therefore, it is catalysed by a base and the generated cyanide ion (CN–) being a stronger nucleophile readily adds to carbonyl compounds to yield corresponding cyanohydrin.
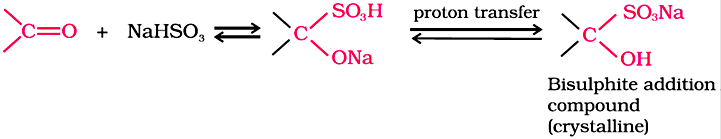
Nucleophilic addition of sodium hydrogensulphite:
Sodium hydrogensulphite adds to aldehydes and ketones to form the addition products.
The position of the equilibrium lies largely to the right-hand side for most aldehydes and to the left for most ketones due to steric reasons.
The hydrogensulphite addition compound is water soluble and can be converted back to the original carbonyl compound by treating it with dilute mineral acid or alkali. Therefore, these are useful for separation and purification of aldehydes.
Nucleophilic addition of Grignard reagents:
Alcohols are produced by the reaction of Grignard reagents with aldehydes and ketones.
The first step of the reaction is the nucleophilic addition of Grignard reagent to the carbonyl group to form an adduct. Thereafter, hydrolysis of the adduct yields an alcohol.

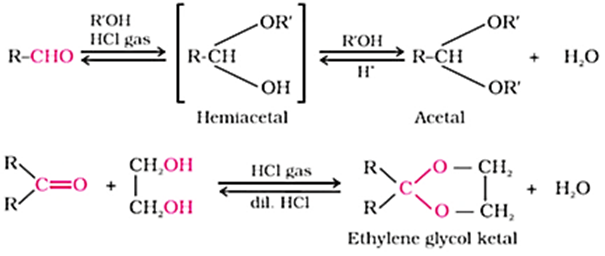
Nucleophilic addition of alcohols:
Aldehydes react with one equivalent of monohydric alcohol in the presence of dry hydrogen chloride to yield alkoxyalcohol intermediate, known as hemiacetals, which further react with one more molecule of alcohol to give a gem-dialkoxy compound known as acetal as shown in the reaction.
Ketones react with ethylene glycol under similar conditions to form cyclic products known as ethylene glycol ketals.
Dry hydrogen chloride protonates the oxygen of the carbonyl compounds and therefore, increases the electrophilicity of the carbonyl carbon facilitating the nucleophilic attack of ethylene glycol.
Acetals and ketals are hydrolysed with aqueous mineral acids to yield corresponding aldehydes and ketones respectively.
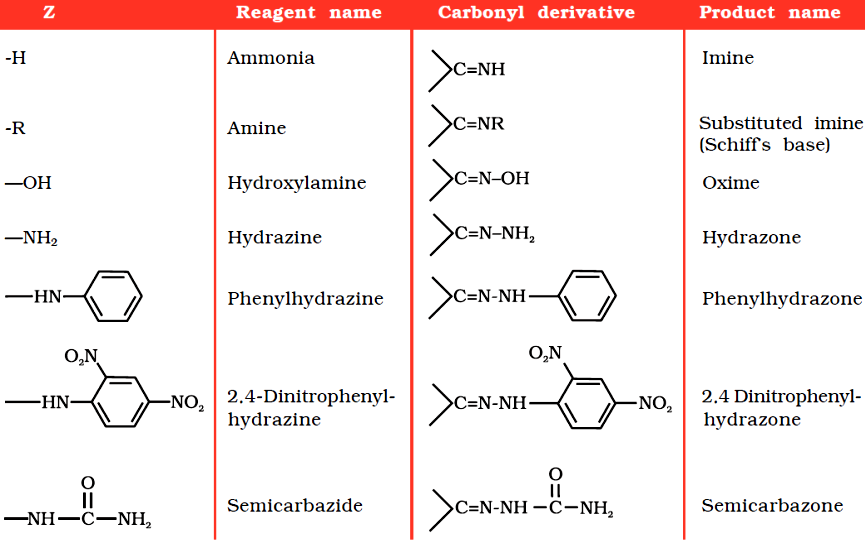
Nucleophilic addition of ammonia and its derivatives:
Nucleophiles, such as ammonia and its derivatives H2N-Z add to the carbonyl group of aldehydes and ketones. The reaction is reversible and catalysed by acid.
The equilibrium favours the product formation due to rapid dehydration of the intermediate to form >C=N-Z.

Some N-Substituted Derivatives of Aldehydes and Ketones (>C=N–Z):
Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminium hydride (LiAlH4) as well as by catalytic hydrogenation.
![]()

(ii) Reduction to hydrocarbons
(a) Clemmensen reduction
![]()
(b) Wolff-Kishner reduction

Aldehydes differ from ketones in their oxidation reactions.
Aldehydes are easily oxidised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate, etc.
Even mild oxidising agents, mainly Tollens’ reagent and Fehlings’ reagent also oxidise aldehydes.
![]()
Ketones are generally oxidised under vigorous conditions, i.e., strong oxidising agents and at elevated temperatures.
Ketones’ oxidation involves carbon-carbon bond cleavage to afford a mixture of carboxylic acids having lesser number of carbon atoms than the parent ketone.
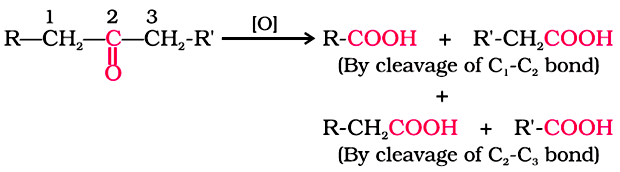
The mild oxidising agents given below are used to distinguish aldehydes from ketones:
On warming an aldehyde with freshly prepared ammoniacal silver nitrate solution (Tollens’ reagent), a bright silver mirror is produced due to the formation of silver metal.
The aldehydes are oxidised to corresponding carboxylate anion.
The reaction occurs in alkaline medium.
![]()
Fehling reagent comprises of two solutions, Fehling solution A and Fehling solution B.
Fehling solution A is aqueous copper sulphate and Fehling solution B is alkaline sodium potassium tartarate (Rochelle salt). These two solutions are mixed in equal amounts before test.
On heating an aldehyde with Fehling’s reagent, a reddish-brown precipitate is obtained.
Aldehydes are oxidised to corresponding carboxylate anion.
Aromatic aldehydes do not respond to this test.
![]()
Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom (methyl ketones) are oxidised by sodium hypohalite to sodium salts of corresponding carboxylic acids having one carbon atom less than that of carbonyl compound.
The methyl group is converted to haloform.
This oxidation does not affect a carbon-carbon double bond, if present in the molecule.
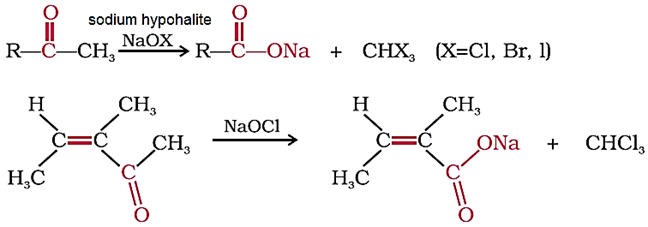
Iodoform reaction with sodium hypoiodite is used for detection of CH3CO group.
Iodoform reaction with sodium hypoiodite is also used for detection of CH3CH(OH) group which produces CH3CO group on oxidation.
4. Reactions due to α-hydrogen
The aldehydes and ketones undergo a number of reactions due to the acidic nature of α-hydrogen.
The acidity of α-hydrogen atoms of carbonyl compounds is due to the strong electron withdrawing effect of the carbonyl group and resonance stabilisation of the conjugate base.

The name aldol is derived from the names of the two functional groups, aldehyde and alcohol, present in the products.
Aldol ≡ Aldehyde and alcohol
Aldehydes and ketones having at least one α-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to form β-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), respectively. This is known as Aldol reaction.
Though ketones give ketols, the general name aldol condensation still applies to the reactions of ketones.
The aldol and ketol readily lose water to give α, β-unsaturated carbonyl compounds which are aldol condensation products.

(ii) Cross aldol condensation:
When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation.
If both of them contain α-hydrogen atoms, it gives a mixture of four products. This is illustrated below by aldol reaction of a mixture of ethanal and propanal.

Ketones can also be used as one component in the cross-aldol reactions.

Aldehydes which do not have an α-hydrogen atom, undergo self-oxidation and reduction (disproportionation) reaction on heating with concentrated alkali.
In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.

6. Electrophilic substitution reaction
Aromatic aldehydes and ketones undergo electrophilic substitution at the ring in which the carbonyl group acts as a deactivating and meta-directing group.

The carboxyl group, consists of a carbonyl group (–CO) attached to a hydroxyl group (–OH), hence its name carboxyl.
Carboxylic acids may be aliphatic (RCOOH) or aromatic (ArCOOH).
In carboxylic acids, the bonds to the carboxyl carbon lie in one plane and are separated by about 120°. The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure shown below:

IUPAC Nomenclature of Carboxyl Group
In the IUPAC system, aliphatic carboxylic acids are named by replacing the ending –e in the name of the corresponding alkane with –oic acid.
In numbering the carbon chain, the carboxylic carbon is numbered one.
For naming compounds containing more than one carboxyl group, the ending –e of the alkane is retained.
The number of carboxyl groups are indicated by adding the multiplicative prefix, di, tri, etc. to the term oic.
Position of –COOH groups are indicated by the numeral before the multiplicative prefix.
Some of the carboxylic acids along with their common and IUPAC names are listed in Table below.
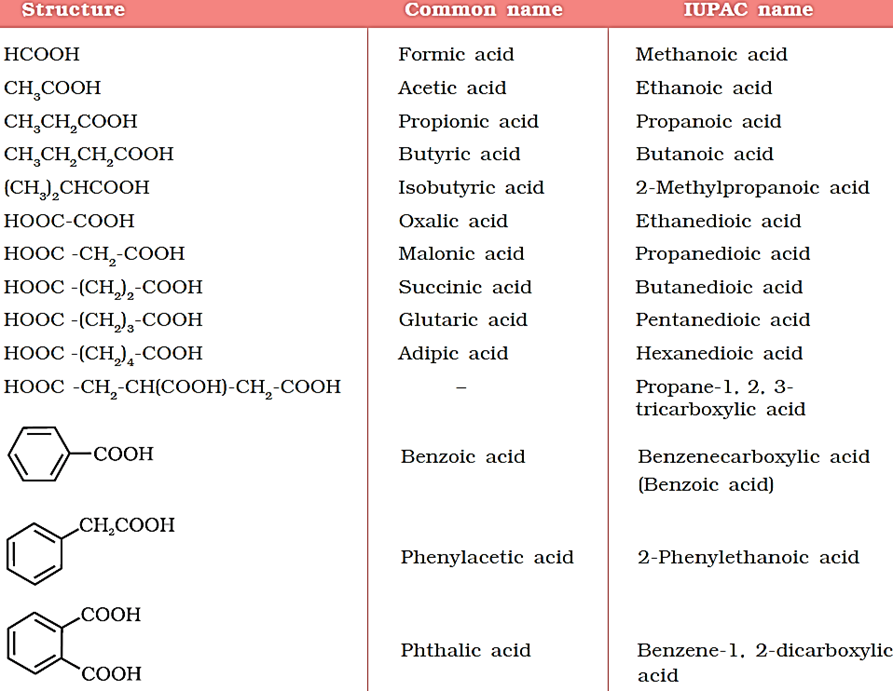
Methods of Preparation of Carboxylic Acids
1. From primary alcohols and aldehydes
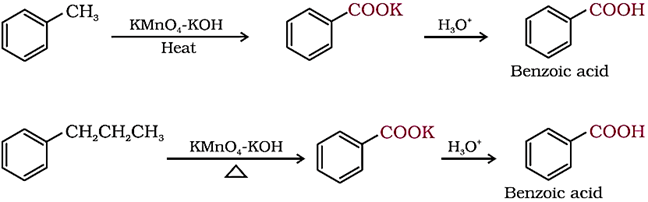
2. From alkylbenzenes
3. From nitriles and amides
4. From Grignard reagents
5. From acyl halides and anhydrides
6. From esters
1. From primary alcohols and aldehydes
Primary alcohols are readily oxidised to carboxylic acids with common oxidising agents such as potassium permanganate (KMnO4) in neutral, acidic or alkaline media or by potassium dichromate (K2Cr2O7) and chromium trioxide (CrO3) in acidic media.

Aldehydes are easily oxidised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate, etc.
Even mild oxidising agents, mainly Tollens’ reagent and Fehlings’ reagent also oxidise aldehydes.
![]()
Aromatic carboxylic acids can be prepared by vigorous oxidation of alkyl benzenes with chromic acid or acidic or alkaline potassium permanganate.
The entire side chain is oxidised to the carboxyl group irrespective of length of the side chain.
Primary and secondary alkyl groups are oxidised in this manner while tertiary group is not affected.
Nitriles are hydrolysed to amides and then to acids in the presence of H+ or OH− as catalyst.
Mild reaction conditions are used to stop the reaction at the amide stage.
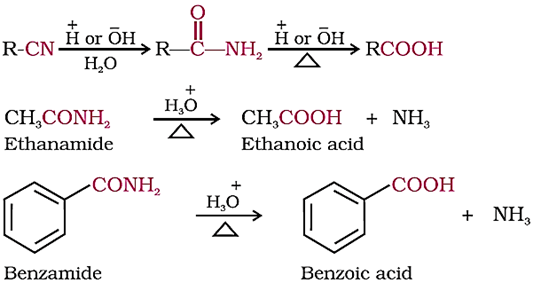
We know, that the nitriles can be prepared from alkyl halides. Hence this method is useful for converting alkyl halides into corresponding carboxylic acids having one carbon atom more than that present in alkyl halides (ascending the series).
Grignard reagents react with carbon dioxide (dry ice) to form salts of carboxylic acids which in turn give corresponding carboxylic acids after acidification with mineral acid.

We know, that the Grignard reagents can be prepared from alkyl halides. Hence this method is useful for converting alkyl halides into corresponding carboxylic acids having one carbon atom more than that present in alkyl halides (ascending the series).
6. From acyl halides and anhydrides
Acid chlorides when hydrolysed with water give carboxylic acids or more readily hydrolysed with aqueous base to give carboxylate ions which on acidification provide corresponding carboxylic acids.

Anhydrides on the other hand are hydrolysed to corresponding acid(s) with water.

Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give corresponding carboxylic acids.

Physical Properties of Carboxylic Acids
Aliphatic carboxylic acids upto nine carbon atoms are colourless liquids at room temperature with unpleasant odours.
The higher acids are wax like solids and are practically odourless due to their low volatility.
Carboxylic acids are higher boiling liquids than aldehydes, ketones and even alcohols of comparable molecular masses.
This is due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding.
The hydrogen bonds are not broken completely even in the vapour phase. In fact, most carboxylic acids exist as dimer in the vapour phase or in the aprotic solvents.

Simple aliphatic carboxylic acids having upto four carbon atoms are miscible in water due to the formation of hydrogen bonds with water. The solubility decreases with increasing number of carbon atoms. Higher carboxylic acids are practically insoluble in water due to the increased hydrophobic interaction of hydrocarbon part.

Chemical Reactions of Carboxylic Acids
1. Reactions Involving Cleavage of O–H Bond
2. Reactions Involving Cleavage of C–OH Bond
(i) Formation of anhydride
(ii) Esterification
(iii) Reactions with PCl5, PCl3 and SOCl2
(iv) Reaction with ammonia
3. Reactions Involving –COOH Group
(i) Reduction
(ii) Decarboxylation
4. Substitution Reactions in the Hydrocarbon Part
(i) Halogenation
(ii) Ring substitution
1. Reactions Involving Cleavage of O–H Bond
(a) The carboxylic acids like alcohols evolve hydrogen with electropositive metals and form salts with alkalies similar to phenols.
However, unlike phenols they react with weaker bases such as carbonates and hydrogencarbonates to evolve carbon dioxide.
This reaction is used to detect the presence of carboxyl group in an organic compound.

(b) Carboxylic acids dissociate in water to give resonance stabilised carboxylate anions and hydronium ion.
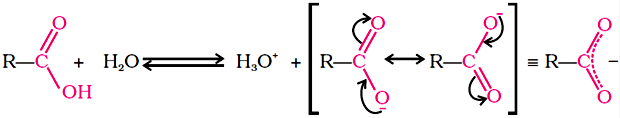
Acidity of Carboxylic Acids
Carboxylic acids are amongst the most acidic organic compounds you have studied so far.
Order of acidity:
Mineral acids > Carboxylic acids > Simple phenols > Alcohols
Higher acidity of carboxylic acids
The conjugate base of carboxylic acid, a carboxylate ion, is stabilised by two equivalent resonance structures in which the negative charge is at the more electronegative oxygen atom.
The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom.
Therefore, resonance in phenoxide ion is not as important as it is in carboxylate ion.
Further, the negative charge is delocalised over two electronegative oxygen atoms in carboxylate ion whereas it is less effectively delocalised over one oxygen atom and less electronegative carbon atoms in phenoxide ion.
Thus, the carboxylate ion is more stabilised than phenoxide ion, so carboxylic acids are more acidic than phenols.
Effect of substituents on the acidity of carboxylic acids
Substituents may affect the stability of the conjugate base and thus, also affect the acidity of the carboxylic acids.
Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects.
Conversely, electron donating groups decrease the acidity by destabilising the conjugate base.

A few groups in increasing acidity order
Ph < I < Br < Cl < F < CN < NO2 < CF3
Direct attachment of groups such as phenyl or vinyl to the carboxylic acid, increases the acidity of corresponding carboxylic acid, contrary to the decrease expected due to resonance effect shown below:
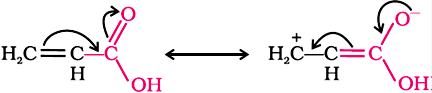
This is because of greater electronegativity of sp2 hybridised carbon to which carboxyl carbon is attached.
The presence of electron withdrawing group on the phenyl of aromatic carboxylic acid increases their acidity while electron donating groups decrease their acidity.

2. Reactions Involving Cleavage of C–OH Bond

![]()
(iii) Reactions with PCl5, PCl3 and SOCl2

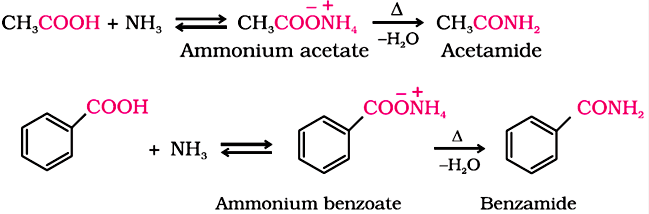
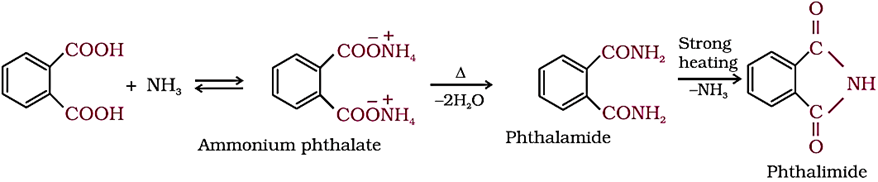
3. Reactions Involving –COOH Group
![]()
(a)
![]()
(b) Alkali metal salts of carboxylic acids also undergo decarboxylation on electrolysis of their aqueous solutions and form hydrocarbons having twice the number of carbon atoms present in the alkyl group of the acid. The reaction is known as Kolbe electrolysis.
2CH3COOK → 2CH3COO– + 2K+
At anode: 2CH3COO– ![]() CH3–CH3 + 2CO2
CH3–CH3 + 2CO2
Ethane
At cathode: 2K+ ![]() 2K
2K ![]() 2KOH + H2 ↑
2KOH + H2 ↑
4. Substitution Reactions in the Hydrocarbon Part
(i) Halogenation (Hell-Volhard-Zelinsky reaction)

Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing group.
They however, do not undergo Friedel-Crafts reaction (because the carboxyl group is deactivating and the catalyst AlCl3 (Lewis acid) gets bonded to the carboxyl group).


Online & Classroom Coaching for Grade 6 to 12 & JEE / NEET
