The elements in which the last electron enters the outermost p-orbital are known as p-block elements.
General electronic configuration: ns2 np1-6 (except He)
The first member, of each group of p-block elements (i.e. elements of the second period) has some properties in common with the heavier elements of their group, due to similarity in outer electronic configuration. However, they show anomalous behaviour in many respects due to their smaller size and the non-availability of d-orbitals.
Elements belonging to the s and p-blocks in the periodic table are called the representative group or elements of main group elements.
The tendency of ns2 electron pair to participate in bond formation decreases with the increase in atomic size.
Within a group the higher oxidation state becomes less stable with respect to the lower oxidation state as the atomic number increases. This trend is called 'inert pair effect'. In other words, the energy, required to unpair the electrons, is more than energy released in formation of two additional bonds.
Variation of properties of group 13 and group 14 elements(Group variation from top to bottom on moving down a Group 13 and 14)
Atomic Radii: Generally increases.
Reason: This is due to continuous increase in the number of electronic shells (principal energy levels).
Exceptions: Atomic radius of Ga is less than that of Al due to the presence of poor shielding of d-electrons in gallium.
Atomic radius increases from C to Si. There is a slight increase in radius from Si to Pb due to presence of completely filled d and f-orbitals.
Ionisation Potential (I.E.): Generally decreases due to increase in the atomic radius of the elements down a group.
Reason: As atomic size (radius) increases -from top to bottom in a group, naturally the distance of the outermost electrons from the nucleus increases and as such the attractive force of nucleus on outermost electrons goes on decreasing. Consequently, the energy required to remove the electron decreases.
Exceptions: (i) I.E. (Ga) > I.E. (Al) and (ii) I.E. (Tl) > I.E. (In) due to the presence of poor shielding d-electrons and f-electrons in Ga and in Tl. I.E. decreases slightly from Si to Ge to Sn and then increases slightly from Sn to Pb due to the presence of poor shielding d and f-electrons.
Electronegativity (En): Generally decreases down a group.
Reason:
![]()
Since both I.E. and E.A. decrease down a group, electronegativity also decreases.
Exceptions: Electronegativity first decreases from B to AI and then increases from Ga to Tl. Si and Pb have almost same value of electronegativity.
Electropositivity: Generally increases down a group.
Explanation: It is due to decrease in the I.E. of the elements on moving down a group.
Metallic Character: Generally increases.
Reason: This is because of increase in atomic size and hence decrease in the ionisation energy of the elements in a group from top to bottom.
Note: Trend of change in metallic character in a group can also be explained on the basis of decreasing trend of electronegativity of the elements down a group.
Non-metallic Character: Generally decreases down a group.
Reason: As electronegativity of elements decreases from top to bottom in a group, nonmetallic character decreases in going down a group.
Basic Nature of Oxides: Generally increases.
Reason: Since metallic character or electropositivity of elements increases in going from top to bottom in a group, basic nature of their oxides naturally increases.
Acidic Nature of Oxides: Generally decreases.
Reason: As non-metallic character of elements decreases in going from top to bottom in a group.
Basic Nature of Hydroxides: It follows the same trend as that of basic nature of oxides for the same reason. (Generally increases).
Acidic Nature of Hydroxides: In general, it follows the same trend as that of acidic nature of oxides for the same reason. (Generally decreases).
Hydrides: Group 13 elements form hydrides of MH3 and group 14 forms MH4 type whose thermal stability decreases as we go down the group.
Reason: It is due to decrease in bond dissociation energy as bond length increases Hydrides of group 13 are weak Lewis acids e.g., BH3, AlH3, GaH3 They form complex hydrides like Li[AlH4], Li[BH4], Li[GaH4].
Reducing Power of Elements: Generally increases down the group.
Reason: Since the I.E. of elements decreases down a group, their reducing power (tendency to lose electrons) increases.
Lattice Energy of Elements: Generally decreases due to increase in atomic radius down a group.
Explanation: The larger the size (radius) of an atom, the lower is the value of its lattice energy. Since in a group, radii of atoms increase in going down a group and lattice energy decreases in the same direction.
Exceptions: Ga has lower lattice energy than Tl which has higher lattice energy than In.
M.P. of Elements: First decreases from B to Ga and then increases.
B.P. of Elements: Generally decreases down the group.
Volatility of Elements: First increases from B to Ga and then decreases.
Hydration Energy of Elements (ions): Generally decreases in going down a group.
Reason: This is due to increase in size (radius) of ions in going down a group.
Density of Metals and Non-metals: Generally increases.
Reason: This is due to more rapid increase in the atomic masses of elements than in their atomic volume.
Comparison of properties of group 13 elements with group 14 elements(From group 13 to group 14 along a period)
Valency: Group 13 elements show the valency of 1 and 3 while group 14 elements show the valency of 2 and 4.
Atomic Radii: Generally decreases due to increase in effective nuclear charge from group 13 to group 14 across a period.
Explanation: From left to right across a period orbit number remains constant and effective nuclear charge increases progressively. Consequently, relative pull (attractive force) of the nucleus on outermost electrons becomes more and more and so atomic size decreases.
First Ionisation Energy (1st I.E.): In general, 1st I.E. of elements increases across a period because of increase in effective nuclear charge and decrease in atomic radii from left to right in a period.
Explanation: As the effective nuclear charge increases and atomic size (radius) decreases across a period, attractive force of nucleus on outermost electrons becomes more and more and so energy required to remove the electron becomes greater and consequently ionisation energy increases from left to right across a period.
Electronegativity (En): Generally increases from left to right along a period.
Reason:
![]()
Since both I.E. and E.A. increase along a period, electronegativity increases also.
Electropositivity of Elements: Generally decreases across a period.
Reason: It is due to increase in the I.E. of elements from left to right in a period.
Metallic Character: Decreases across a period.
Reason: It is as a result of decrease in atomic size and hence increases in the I.E. of elements along a period from left to right.
Non-metallic Character: Increases along a period.
Reason: As electronegativity of elements increases from left to right in a period, nonmetallic character also increases in the same direction.
Basic Nature of Oxides: Decreases from left to right in a period.
Reason: Since electropositivity and hence metallic character of elements decreases along a period, basic nature of their oxides naturally decreases.
Acidic Nature of Oxides: Increases from left to right in a period.
Reason: Since electronegativity and hence nonmetallic character increases along a period; therefore, acidic nature of their oxides naturally increases.
Basic Nature of Hydroxides: In general, it follows the same trend as that of basic nature of oxides for the same reason. (Generally decreases).
Acidic Nature of Hydrides: Generally increases from left to right in a period.
Reason: Non-metallic character increases from left to right, therefore, acidic character of hydrides increases e.g., LiH is basic, H2S and HF are acidic.
Basic Nature of Hydrides: Generally decreases.
Reason: Since acidic nature of hydrides increases from left to right in a period, basic nature of hydrides naturally decreases.
Reducing Power of Hydrides: Generally decreases along a period.
Reason: Due to increase in the electronegativity of elements from left to right in a period.
Examples: In the 2nd period, LiH is the strongest reducing agent and HF is the weakest reducing agent.
The Boron FamilyGroup 13 elements boron (B), aluminium (Al), gallium (Ga), indium (In) and thallium (Tl) belong to boron family. They have general electronic configuration ns2np1. They belong to p-block of periodic table.
Ores of Boron:
(i) Borax, Na2B4O7.10H2O
(ii) Kernite, Na2B4O7.2H2O
(iii) Colemanite, Ca2B6O11.5H2O
Boron(i) Its atomic number is 5 and mass no. is 11.
(ii) Its electronic configuration is 1s22s22p1.
(iii) It has 3 valence electrons, and it belongs to group 13.
(iv) Boron forms covalent compounds due to smallest size and highest ionisation energy.
(v) It resembles silicon due to same charge/radius ratio.
(vi) It forms electron deficient compounds, e.g. BCl3, BF3, BBr3, BI3 which are called Lewis acids because they can easily accept electrons from the donor species, e.g.
Physical Properties:(i) It is extremely hard solid due to strong covalent bond which is due to small atomic size.
(ii) It has high m.pt. (2450 K) and low electrical conductivity.
(iii) It has four allotropes. Crystalline boron is black and chemically inert. It is very hard in nature. Amorphous boron is brown and chemically active. The transition between different forms is extremely slow process.
(iv) It is poor conductor of heat and electricity.
(v) It has two isotopes: ![]() and
and ![]()
(vi) Its density is 2.45 g/ml.
Chemical Properties:(i) It is less reactive at room temperature towards most of the reagents. It reacts with strong oxidising agents at room temperature such as F2 and cone. HNO3 and conc. H2SO4.
2B + 3F2 → 2BF3
B + 3HNO3 (conc.) → H3BO3 + 3NO2
2B + 3H2SO4 (conc.) → 2H3BO3 + 3SO2
(ii) It burns in presence of O2 to form B2O3 and with N2 to form BN.
4B + 3O2 → 2B2O3
2B + N2 → 2BN
(iii)
2B + 3H2O (steam) → B2O3 + 3H2
Red hot Boron trioxide
(iv)
2B + 6NaOH ![]() 2Na3BO3 + 3H2
2Na3BO3 + 3H2
(v)
3Mg + 2B → Mg3B2
(vi)
4B + 3CO2 → 2B2O3 + 3C
(vii)
3SiO2 + 4B → 2B2O3 + 3Si
(viii)
2B + 3Cl2 → 2BCl3
Compounds of BoronBoranes: The series of compounds of boron with hydrogen is known as boranes. Two series of boranes, BnHn + 4 and BnHn + 6 are particularly important. The simplest form of borane is diborane (B2H6) which is used as reducing agent.
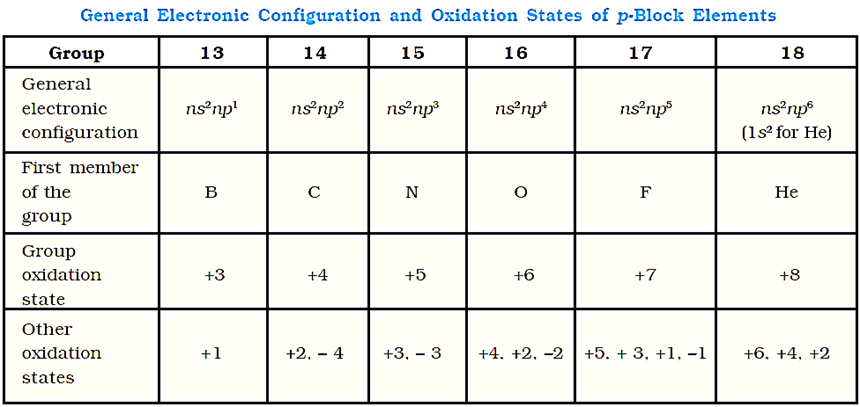
BH3 does not exist because it is electron deficient. It exists in the form of dimer B2H6. The four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below the planar, there are two bridging H-atoms. The terminal B-H bonds are regular bonds but the bridge B-H bonds are different and can be described in terms of three centre two electron bonds shown below:
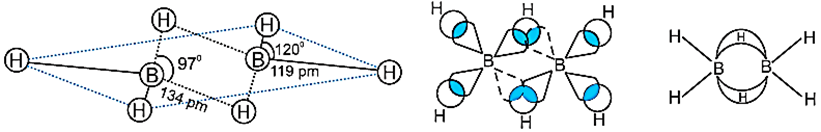
Methods of Preparation of Diborane:
(i) BF3 is treated with LiAlH4 in ether.
4BF3 + 3LiAlH4 → 2B2H6 + 3LiF + 3AlF3
(ii) Oxidation of NaBH4 with I2
2NaBH4 + I2 → B2H6 + 2NaI + H2
(iii) It is also prepared by reaction of BF3 with sodium hydride on industrial scale.
2BF3 + 6NaH ![]() B2H6 + 6NaF
B2H6 + 6NaF
The higher boranes are formed when B2H6 is heated at 373 K-523 K.
Properties of Diborane:(i) It is colourless toxic gas.
(ii) It has boiling point of 180 K.
(iii) It catches fire spontaneously upon exposure to air. It burns in oxygen releasing an enormous amount of energy.
B2H6 + 3O2 → B2O3 + 3H2O ∆H = –1976 kJ
(iv) B2H6 is used as rocket fuel. Most of higher boranes are also spontaneously flammable in air. Boranes are readily hydrolysed by water to give boric acid.
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2(g)
(v) Diborane undergoes cleavage reactions with Lewis bases to give borane adducts.
B2H6 + 2N(CH3)3 → 2BH3.N(CH3)3
Diborane Trimethyl amine
B2H6 + 2CO → 2BH3.CO
(vi) Reaction of ammonia with diborane gives initially B2H6.2NH3 formulated as [BH2(NH3)2)+[BH4]-; further heating gives borazine, B3N3H6 known as 'inorganic benzene' in view of its ring structure with alternate BH and NH groups. It is also called borazole.
3B2H6 + 6NH3 → 3[BH2(NH3)2]+[BH4]–
![]() 2B3N3H6 + 12H2
2B3N3H6 + 12H2
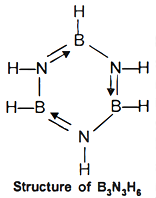
(vii) Boron also forms a series of hydrido borates; the most important one is the tetrahedral [BH4]- ion. Li and Na tetrahydrido borates, also known as borohydrides, are prepared by reaction of metal hydride with B2H6 in diethyl ether (solvent).
2NaH + B2H6 → 2[Na]+[BH4]–
Sodium borohydride
2LiH + B2H6 → 2LiBH4
Lithium borohydride
(Lithium tetrahydroborate)
Uses of diborane:(i) Both LiBH4 and NaBH4 are used as reducing agents in organic synthesis.
(ii) They are also used for preparing other metal borohydrides.
(b) BoraxBoron forms strong bonds with oxygen
in borates.
Borax dissolves in water to give alkaline solution.
Na2B4O7 + 2H2O → 2NaOH + H2B4O7
Borax is therefore used as a water softener and cleansing agent.
On heating, hydrated borax loses water giving anhydrous spongy mass which contracts to a colourless glass like solid. At about 1000 K, sodium metaborate and boron oxide are formed.
Na2B4O7 ![]() 2NaBO2 + B2O3
2NaBO2 + B2O3
It is trivial name for orthoboric acid (H3BO3). It is usually obtained by acidifying an aqueous solution of borax.
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4H3BO3
Boric acid is also obtained by hydrolysis of BCl3.
BCl3 + 3H2O → H3BO3 + 3HCl
Properties of Boric acid:(i) It is white crystalline solid.
(ii) It has layer structure in which planar BO3 units are joined by H-bonds.
(iii) It is soluble in water. It is weak monobasic acid. It acts as Lewis acid by accepting a hydroxy lion.
B(OH)3 + H2O → [B(OH)4]– + H+
(iv) It forms metaboric acid on heating at 370 K.
H3BO3  HBO2 + H2O
HBO2 + H2O
(v) It forms B2O3 above 373 K.
2HBO2  B2O3 + H2O
B2O3 + H2O
(i) Borax is used to make heat resistant glass.
(ii) Borax is used as a flux for soldering metals.
(iii) A dilute solution of boric acid is used as mild antiseptic.
(iv) Boron ![]() is
used to absorb neutrons, metal borides are used in nuclear reactors as control
rods.
is
used to absorb neutrons, metal borides are used in nuclear reactors as control
rods.
(v) Boron is used in making light composite materials for aircrafts.
(vi) Borax is used in borax bead test and for making porcelain enamels.
Borax Bead TestColoured salts of metals, on heating with borax form coloured glass like bead from which these metals can be identified due to the formation of metal metaborate e.g. cobalt forms blue bead, chromium forms green bead.
Uses of Aluminium and its Compounds:(i) Aluminium is used in making duralumin which is used for making parts of aircraft, utensils.
(ii) Potash alum is used as an antiseptic and also used in transportation industry.
(iii) AlCl3 is used as a catalyst.
Carbon FamilyGroup 14 elements - carbon (C),
silicon (Si), germanium (Ge), tin (Sn) and lead (Pb) belong to carbon family.
The general electronic configuration of carbon family is ns2 np2.
(i) Carbon has atomic number 6, mass number 14.
(ii) Its electronic configuration is 1s2 2s2 2px1 2py1.
(iii) It is first member of Group 14. It is typical non-metal forming covalent bonds in the vast majority of its compounds by making use of all its four valence electrons.
(iv) Carbon has a property of self-linking in chains and rings. This property is called catenation.
(v) Carbon can form multiple bonds with itself as well as with other elements particularly nitrogen and oxygen.
Allotropy
It is a phenomenon due to which certain elements exist in different forms having different physical properties
but similar chemical properties, e.g.,
carbon has different allotropes:
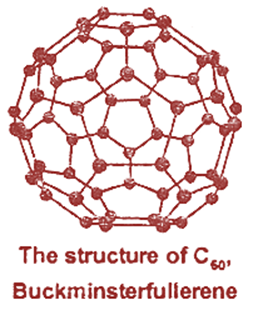
Graphite, Diamond, Fullerene.
Fullerenes
Fullerenes were originally made by the evaporation of graphite using
a laser. A more practical method involves the heating of graphite in electric
arc in presence of an inert gas such as helium or argon. The sooty material
formed by condensation of vapourised en small molecules consist of mainly C60
with smaller quantity of C70 and traces of other fullerenes consist
of even number of carbon atoms upto 350 or above. C60 and C70
can be readily separated from fullerene soot by extraction in toluene followed
by chromatographic separation over alumina. The molecules have the shape of
soccer ball (football).
Water Gas
It is mixture of carbon monoxide and
hydrogen. It is obtained by passing steam over red hot coke. It is used
as an industrial fuel.
Producer Gas
It is a mixture of carbon monoxide and
nitrogen. It is used for heating glass retorts (apparatus). It is prepared
by passing air over red hot coke.
Dry Ice
Solid CO2 is known as
dry ice. It is obtained by cooling CO2 under pressure. It is called
dry ice because it sublimes (changes into vapour directly without becoming
liquid).
Freon-12
It is dichlorodifluoro methane, CF2Cl2.
It is used as a refrigerant.
Carborundum
It is silicon carbide, SiC. It is
hard and used as abrasives (for grinding surfaces).
Catenation
It is phenomenon of an atom to form a strong covalent bond with the
atoms of itself. Carbon shares the property of catenation of maximum extent
because it is small in size and can form pπ-
pπ multiple bonds to itself.
Tendency to show catenation decreases down the group 14 due to decrease in bond
dissociation energy which is due to increase in atomic size.
Occurrence of Carbon
It is widely distributed in the form of coal, petroleum and metal
carbonates of electropositive metals.
All living systems contain carbon compounds. Carbon is also present as CO2
in atmosphere.
Isotopes of Carbon
Naturally occurring carbon has two
stable isotopes ![]() (98.9%) and
(98.9%) and ![]() (1.1%) in addition to traces of radioactive
(1.1%) in addition to traces of radioactive ![]() isotope with half-life 5770 years. It is used in
radioactive carbon dating to determine the age of archaeological specimens of
organic origin. The isotope
isotope with half-life 5770 years. It is used in
radioactive carbon dating to determine the age of archaeological specimens of
organic origin. The isotope ![]() is the international standard for atomic mass
measurement and assigned a mass of 12.00000 units.
is the international standard for atomic mass
measurement and assigned a mass of 12.00000 units.
The Oxides of Carbon: There are two well-known oxides of carbon, carbon monoxide, CO and carbon dioxide, CO2.
(a) Carbon MonoxideIt is formed by the incomplete combustion of carbon or carbon containing fuels. It is present in automobile exhaust gases.
C(s) + ![]() O2(g) → CO(g)
O2(g) → CO(g)
The pure carbon monoxide is prepared on smaller scale by dehydrating (removing water) pure formic acid with cone. H2SO4.
HCOOH ![]() CO + H2O
CO + H2O
Commercial Method:
CO is commercially produced by the passage of steam over red hot
coke. We get mixture of CO and H2. It is called water gas or
synthesis gas.
C + H2O → CO + H2
Steam (Water gas)
Preparation of Producer Gas:
When air is passed instead of steam over heated coke, a mixture of CO and N2 are produced which is called producer gas.
2C + O2 + 4N2 → 2CO + 4N2
( air ) ( Producer gas )
Properties:(i) Carbon monoxide is a colourless, odourless gas.
(ii) It is extremely poisonous gas because it combines with haemoglobin to form carboxyhaemoglobin which does not act as oxygen carrier.
(iii) Carbon monoxide is a powerful reducing agent and reduces metal oxides to the corresponding metals.
Fe2O3 + 3CO ![]() 2Fe + 3CO2
2Fe + 3CO2
(iv) It readily combines with Cl2 in presence of sunlight to give carbonyl chloride (phosgene gas).
CO + Cl2 → COCl2
(v) Carbon monoxide also combines with some transition metals to form gaseous carbonyls e.g. [Ni(CO)4].
Ni + 4CO → Ni(CO)4
Fe + 5CO → Fe(CO)5
Iron pentacarbonyl
Cr + 6CO → Cr(CO)6
Chromium hexacarbonyl
(b) Carbon Dioxide Methods of Preparation:
(i) It is formed by the combustion of carbon and other fossil fuels in an excess of oxygen.
C(s) + O2(g) → CO2(g)
C5H12(l) + 😯2(g) → 5CO2(g) + 6H2O(l)
(ii) Commercially CO2 is produced by heating limestone.
CaCO3(s) ![]() CaO + CO2
CaO + CO2
In the laboratory, CO2 is produced by the action of acids on· carbonates.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Carbon dioxide is also formed during fermentation.
Properties:(i) Carbon dioxide is a colourless gas.
(ii) It is heavier than air with a density of 1.977 g/L at 273 K.
(iii) CO2 is non-poisonous but does not support life in humans or animals. It can cause suffocation and eventually death on account of lack of oxygen.
(iv) It is soluble in water. The solubility increases with increase in pressure. Soda water and other aerated soft drinks are solution of CO2 in water under pressure.
(v) It is weakly acidic oxide. When it dissolves in water, only some molecules react with water to form carbonic acid, H2CO3. H2CO3 is weak dibasic acid. It forms two series of salts, the carbonates and hydrogen carbonates (bicarbonates). CO2 is anhydride of carbonic acid.
CO2(g) + H2O(l) → H2CO3(aq)
H2CO3(aq) ⇌ H+(aq) + HCO3–(aq)
HCO3–(aq) ⇌ H+(aq) + CO32–(aq)
Thus, a solution of CO2 in water is in an equilibrium mixture of
CO2, H2CO3, HCO3–, CO32–.
Uses of CO2:(i) CO2 is used as refrigerant.
(ii) Solid CO2 can sublime to the gaseous state without passing through the liquid stage and hence is called dry ice.
(iii) Dry ice is used as coolant for preserving ice-cream and perishable food.
(iv) Dry ice is also used as cooling agent for carrying out reactions below 273 K.
(v) Carbon dioxide is also used in fire-extinguisher.
(c) Silicon Dioxide, SiO2SiO2 is commonly called silica. Its crystalline forms are quartz, cristobalite and tridymite. They are interconvertible at suitable temperature. It is covalent, three-dimensional network in which each Si atom is covalently bonded to four oxygen atoms tetrahedrally.
Properties:
(i) It is less reactive due to high bond dissociation energy of Si-O bond.
(ii) It does not react with halogens, hydrogen, most of the acids and metals even at high temperature but it reacts with HF and NaOH.
SiO2 + 2NaOH → Na2SiO3 + H2O
SiO2 + 4HF → SiF4 + 2H2O
Uses:(i) It is used in piezoelectric material in form of quartz.
(ii) It is also used in quartz watches.
(iii) Silica gel is used in chromatography and also as a drying agent.
(d) SiliconesSilicones are synthetic polymers containing repeated units of R2SiO where 'R' is alkyl group. They are called silicones because their general formula is similar to ketone, R2CO. They can be made into rubbery elastomers and resin. They are heat resistant and water-repellent.
(e) SilicatesThey are complex network solids in which silicon is usually co–ordinated tetrahedrally bonded to oxygen atoms. They have complex structure due to sharing of one or more oxygen atoms between SiO4 tetrahedra. They have chains, rings, sheets and three-dimensional frameworks of SiO4 tetrahedral units arranged in different ways.
(f) ZeolitesZeolites are generally sodium aluminium silicates. Cation can be potassium or calcium also.
Uses:(i) They are used as water softener.
(ii) They are used as catalyst in petrochemical industry e.g., ZSM-5 is used to convert alcohol to petrol.
Online Tuitions & Self-Study Courses for Grade 6 to 12 & JEE / NEET
