Class 12 Chemistry
Chapter 9 – Coordination Compounds
Magical Series Assignment 9.1
Q.1. When a coordination compound CrCl3·6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write IUPAC name of the complex.
A.1: pentaaquachloridochromium (III) chloride.
Q.2. Write the IUPAC name of the complex [Cr(NH3)4Cl2]Cl.
A.2: tetraamminedichloridochromium(III) chloride.
Q.3. Write down the formula of: Tetraamineaquachloridocobalt(III)chloride.
A.3: [Co(NH3)4H2O)(Cl)]Cl2
Q.4. Name the following coordination compound: K3[CrF6]
A.4: Potassium hexafluoridochromate(III).
Q.5. Write the IUPAC name of [PtCl(NH2CH3)(NH3)2]Cl.
A.5: Diamminechlorido(methylamine) platinum(II) chloride.
Q.6. Write the IUPAC name of [Pt(NH3)4Cl2]Cl2.
A.6: Tetraamminedichloridoplatinum(IV) chloride.
Q.7. Write the IUPAC name of [Cr(NH3)6 [Co(CN)6].
A.7: Hexaamminechromium (III) hexacyanocobaltate(III).
Q.8. Write the IUPAC name of [Co(NH3)5Cl]Cl2. ( Atomic no. of Co = 27)
A.8: Pentaamminechloridocobalt(III) chloride.
Q.9. Using IUPAC norms write the formulae for the following:
(a) Sodium dicyanidoaurate(I)
(b) Tetraamminechloridonitrito–Nplatinum(IV) sulphate
A.9: (a) Na[Au(CN)2] Sodium dicyanidoaurate(l)
(b) [Pt(NH3)4Cl)(NO2)]SO4 Tetraamminechloridonitrito–N–platinum(IV) sulphate
Q.10. Using IUPAC norms write the formulae for the following:
(a) Tris( ethane–1 ,2,diamine )chromium(III) chloride
(b) Potassium tetrahydroxozincate(II)
A.10: (a) [Cr(en)3]Cl3 : Tris(ethane–1,2–diamine)chromium(III) chloride
(b) K2[Zn(OH)4] : Potassium tetrahydroxozincate(II)
Q.11. When a coordination compound CoCl3·6NH3 is mixed with AgNO3, 3 moles of AgCl are precipitated per mole of the compound. Write structural formula of the complex.
A.11: Structural formula: [Co(NH3)6]Cl3
Q.12. Write the coordination number and oxidation state of platinum in the complex [Pt(en)2Cl2]
A.12: Coordination number and oxidation state of Pt in the complex [Pt(en)2Cl2] are 6 and +2 because en is a bidentate and neutral ligand.
Q.13. What do you understand by 'denticity of a ligand'?
A.13: Denticity: The number of coordinating groups present in a ligand. For example, bidentate ligand ethane–1, 2–diamine has two donor nitrogen atoms which can link to central metal atom.
![]()
Q.14. Giving a suitable example, explain the following: Ambidentate ligand
A.14: Ambidentate ligand : A unidentate ligand which can coordinate to central metal atom through two different atoms is called ambidentate ligand. For example NO2 ion can coordinate either through nitrogen or through oxygen to the central metal atom/ion.
Q.15. What is meant by chelate effect?
A.15: When a di– or polydentate ligand uses its two or more donor atoms to bind a single metal ion. It is said to be a chelate ligand. Chelating ligands form more stable complexes than monodentate analogs. This is called chelating effect.
Q.16. Write the formula of the coordination compound: Iron (Ill) hexacyanoferrate(II)
A.16: Fe4[Fe(CN)6]3
Q.17. Write the IUPAC name of the following complex: [Cr(NH3)3Cl3].
A.17: triamminetrichloridochromium (III).
Q.18. Write the IUPAC name of the following complex: [Co(NH3)5(CO3)]Cl.
A.18: Pentaamminecarbonatocobalt(III) chloride
Q.19. Using IUPAC norms write the formulae for the following:
(a) Potassium trioxalatoaluminate(III)
(b) D ichlorido bis( ethane–1 ,2– diamine) cobalt(III)
A.19: (a) K3[Al(C2O4)3] : Potassium trioxalatoaluminate(III)
(b) [CoCl2(en)2]+ : Dichloridobis(ethane–1,2–diamine)cobalt(III) ion
Q.20. (i) Write down the IUPAC name of the following complex: [Cr(NH3)2Cl2( en)]Cl
(ii) Write the formula for the following complex: Pentaamminenitrito–O–cobalt (III).
A.20: (i) Diamminedichlorido( ethane–1 ,2–diamine) chromium(III) chloride.
(ii) [Co(NH3)5(ONO)]2+
Q.21. Using IUPAC norms write the formulae for the following coordination compounds:
(i) Hexaamminecobalt(III)chloride
(ii) Potassium tetrachloridonickelate(II)
A.21: (i) [Co(NH3)6]Cl3
(ii) K2[NiCl4]
Q.22. (i) Write down the IUPAC name of the following complex: [Cr(en)3]Cl3.
(ii) Write the formula for the following complex: Potassium trioxalatochromate (III)
A.22: (i) Tris(ethylenediammine)chromium(III) chloride
(ii) K3[Cr(ox)3]
Q.23. Name the following coordination compounds according to IUPAC system of nomenclature:
(i) [Co(NH3)4H2O)Cl]Cl2 (ii) [CrCl2(en)2]Cl
A.23: (i) Tetraammineaquachloridocobalt(III) chloride
(ii) Dichloridobis(ethane–1,2–diamine)chromium(III)chloride
Q.24. Write the IUPAC name of the following:
(i) [Co(NH3)6]Cl3 (ii) [NiCl4]2– (iii) K3[Fe(CN)6]
A.24: (i) Hexaamminecobalt(III) chloride
(ii) Tetrachloridonickelate(II) ion
(iii) Potassium hexacyanoferrate(III)
Q.25. Write down the IUPAC name of [Co(NH3)5Cl]Cl2 .
A.25: Pentaamminechloridocobalt(III) chloride
Q.26. Write the IUPAC name and draw the structure of each of the following complex entities:
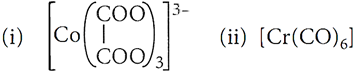
(iii) [PtCl3(C2H4)]–
( At. nos. Cr = 25, Co = 27, Pt = 78)
A.26: (i) Trioxalatocobaltate(III)
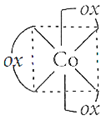
(ii) Hexacarbonylchromium(O)
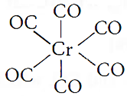
(iii) Trichloroetheneplatinum(III)
Q.27. Write the IUPAC names of the following coordination compounds :
(i) [Cr(NH3)3Cl3] (ii) [CoBr2(en)2]+
A.27: (i) Triamminetrichloridochromium(III)
(ii) Dibromidobis(ethane–1 ,2–diamine )cobalt(III) ion
Q.28. What type of isomerism is exhibited by the complex [Co(NH3)5Cl]SO4?
A.28: Ionisation isomerism: [Co(NH3)5Cl]SO4 and [Co(NH3)5SO4]Cl
Q.29. What type of isomerism is shown by the complex [Co(NH3)6][Cr(CN)6]?
A.29: Coordination isomerism.
Q.30. What type of isomerism is shown by the complex [Co(en)3]Cl3?
A.30: The complex, [Co(en)3]Cl3 shows optical isomerism.
Q.31. What type of isomerism is shown by the complex [Co(NH3)5(SCN)]2+?
A.31: The complex [Co(NH3)5(SCN)]2+ shows linkage isomerism as SCN– is an ambidentate ligand.
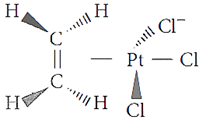
Q.32. Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
A.32: cis–isomer of the complex [Pt(en)2Cl2]2+ is optically active.
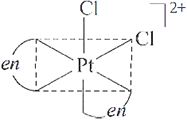
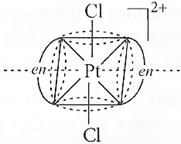
Q.33. Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically inactive.
A.33: Trans–isomer is optically inactive due to the presence of plane of symmetry.
Q.34. Draw the geometrical isomers of complex [Pt(NH3)2Cl2].
A.34:
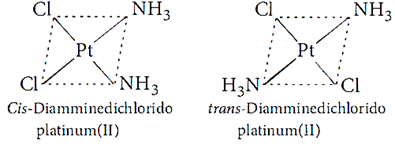
Online Tuitions & Self-Study Courses for Grade 6 to 12 & JEE / NEET
