Chapter 1 – Basic Concepts of Chemistry
Important Questions Answers Set 1
Q.1. How many cm are there in 1 pm?
Q.2. Convert the following into basic units:
(i) 28.7 pm
(ii) 15.15 pm
(iii) 25365 mg
Q.3. Fill in the blanks in the following conversions:
(i) 1 km = .......................................... mm = ...................................... pm
(ii) 1 mg = ........................................... kg = ...................................... ng
(iii) 1 ml = .......................................... L = ...................................... dm3
Q.4. Calculate the number of He atoms in
(i) 52 u
(ii) 52 g
(iii) 52 moles of He.
(Atomic wt. of He is 4 u.)
Q.5. Define the law of conservation of mass.
Q.6. State the law of multiple proportions.
Q.7. State Gay Lussac's law of gaseous volumes.
Q.8. State Avogadro law.
Q.9. What do you mean by significant figures?
Q.10. For an actual result of an observation to be 5; two students A and B reported their readings as follows:
|
|
Observation Reading |
Average |
|
|
|
1 |
2 |
|
|
Student A |
4.95 |
4.93 |
4.94 |
|
Student B |
4.94 |
5.05 |
4.995 |
Which of the students has made a more precise observation? Is his observation accurate too?
Q.11. How many significant figures are present in
(i) 0.0025
(ii) 600.0
Q.12. What is the mass of 1 L of mercury in grams and in kilograms, if the density of liquid mercury is 13.6 g cm−3?
Q.13. Vitamin C is known to contain 1.29 × 1024 hydrogen atoms. Calculate the number of moles of hydrogen atoms.
Q.14. What is the number of significant figures in 0.001620?
Q.15. The height of a person is 155.01 cm. What is the least count of the scale used?
Q.16. Calculate the sum of 7.21, 10.245, 0.0548 in terms of significant figure.
Q.17. What is the SI unit of energy?
Q.18. Express 5.607892 to four significant figures and write the result in standard form.
Q.19. Calculate the percentage of nitrogen in NH3. (Atomic mass of N = 14, H = 1 amu)
Q.20. Calculate molecular mass of glucose (C6H12O6) molecule.
Q.21. Express the following in the scientific notation:
(i) 0.0048
(ii) 234,000
(iii) 8008
(iv) 500.0
(v) 6.0012
Q.22. How many significant figures are present in the following?
(i) 0.0025
(ii) 208
(iii) 5005
(iv) 126,000
(v) 500.0
(vi) 2.0034
Q.23. Round off the following figures upto three significant figures:
(i) 34.216
(ii) 10.4107
(iii) 0.04597
(iv) 2808
Q.24. The following data are obtained when dinitrogen and dioxygen react together to form different compounds:
|
|
Mass of dinitrogen |
Mass of dioxygen |
|
(i) |
14 g |
16 g |
|
(ii) |
14 g |
32 g |
|
(iii) |
28 g |
32 g |
|
(iv) |
28 g |
80 g |
Which law of chemical combination is obeyed by the above experimental data? Give its statement.
Q.25. How many electrons are present in 16 g of CH4?
Answers
A.1: l pm = 10−12 m = 10−10 cm
A.2: (i) 28.7 pm = 28.7 × 10−12 m = 2.87 × 10−11 m
(ii) 15.15 pm = 15.15 × 10−12 m = l .515 × 10−11 m
(iii) 25365 mg = 25365 × 10−6 kg = 2.5365 × 10−2 kg
A.3: (i) l km = 1000 × 1000 mm
= 106 mm = ![]() pm
pm
= 1015 pm [ 1 pm = 10−12 m ]
(ii) 1 mg = 10−6 kg = 106 ng (nanogram)
(iii) 1 ml = 10−3 L = 10−3 dm3
A.4: (i) 4 u is mass of 1 atom
Þ 52 u is mass of ![]() =
13 atoms
=
13 atoms
(ii) 4 g of He contains 6.022 × 1023 He atoms
Þ 52 g of He contains ![]() =
7.8286 × 1024 atoms
=
7.8286 × 1024 atoms
(iii) 1 mole of He contains 6.022 × 1023 atoms 52 moles of He contains 52 × 6.022 × 1023
= 3.131 × 1025 atoms
A.5: Matter can neither be created nor be destroyed.
A.6: Law of multiple proportions: Whenever two elements combine to form two or more compounds, the ratio between different weights of one of the elements which combines with fixed weight of other is always simple whole ratio.
A.7: Whenever gases react, they do so in volume and bear a simple ratio among themselves and to the products if they are gases at same temperature and pressure.
A.8: Equal volumes of all gases contain equal number of molecules at same temperature and pressure.
A.9: Significant figures are meaningful digits which are known with certainty.
A.10: Student 'A' has made a more precise observation since the variation in the two readings taken by him is not much. His observation is precise but is not accurate since his readings are not close to the actual reading which is 5. Student B is more accurate as his average reading is close to actual reading i.e. 5.
A.11: (i) Two, (ii) Four
A.12: Mass = volume × Density
= 1000 cm3 × 13.6 g cm−3 = 13600 g = 13.6 kg
A.13: Number of moles of hydrogen
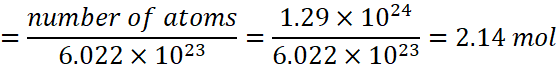
A.14: Four
A.15: 0.01
A.16: 17.5
A.17: joule
A.18: 5.608
A.19:
% of nitrogen in NH3 ![]()
A.20: Molecular mass of glucose
= 6C + 12 H + 6O
= 6 × 12 + 12 × 1 + 6 × 16
= 72 + 12 + 96 = 180 u
A.21: (i) 0.0048 = 4.8 × 10−3
(ii) 234,000 = 2.34 × 105
(iii) 8008 = 8.008 × 103
(iv) 500.0 = 5 × 102
(v) 6.0012 = 6.0012 × 100
A.22: (i) 2 (ii) 3 (iii) 4
(iv) 3 as it should be expressed as 1.26 × 105
(v) 4 (vi) 5
A.23: (i) 34.2
(ii) 10.4
(iii) 0.0460
(iv) 2810
A.24: The given data represents Law of multiple proportions. It states 'whenever two elements combine to form two or more compounds, the ratio between different weights of one of the elements which combines with fixed weight of another is always simple whole ratio.
A.25: 1 molecule of CH4 = 6 + 4 = 10 electrons
16 g of CH4 contains 10 × 6.022 × 1023 electrons = 6.022 × 1024 electrons
Online Tuitions & Self-Study Courses for Grade 6 to 12 & JEE / NEET
