Biomolecules may be defined as complex lifeless
chemical substances which form the basis of life,
i.e., they not only buildup living systems (creatures) but are also responsible
for their growth, maintenance and their ability to reproduce.
The various biomolecules are carbohydrates, proteins, enzymes, nucleic acids, lipids, hormones and compounds for storage and exchange of energy such as adenosine triphosphate (ATP).
Carbohydrates:
These include compounds like sugars, starches, glycogen, cellulose, dextrins and gums.
They are formed in plants by a process known as photosynthesis and make up about 70% of the solid plant material.
Old definition of carbohydrate: (meaning hydrate of carbon)
A class of compounds containing only carbon, hydrogen and oxygen; the hydrogen and oxygen being present in the same ratio as in water, represented by the Cx(H2O)y.
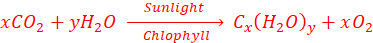
Limitations of the old definition:
(i) A number of compounds such as rhamnose, (C6H12O5) and deoxyribose (C5H10O4) are known which are carbohydrates by their chemical behaviour but cannot be represented as hydrates of carbon.
(ii) There are other substances like formaldehyde (HCHO, CH2O) and acetic acid [CH3COOH, C2(H2O)2] which do not behave like carbohydrates but can be represented by the general formula, Cx(H2O)y.
(iii) Carbon is not known to form hydrates.
Modern definition of carbohydrate:
Carbohydrates are defined as optically active poly hydroxy aldehydes or polyhydroxy ketones or substances which give these on hydrolysis.
Note that aldehydic and ketonic groups in carbohydrates are not present as such but usually exist in combination with one of the hydroxyl groups of the molecule in the form of hemiacetals and hemiketals respectively.
Classification of Carbohydrates
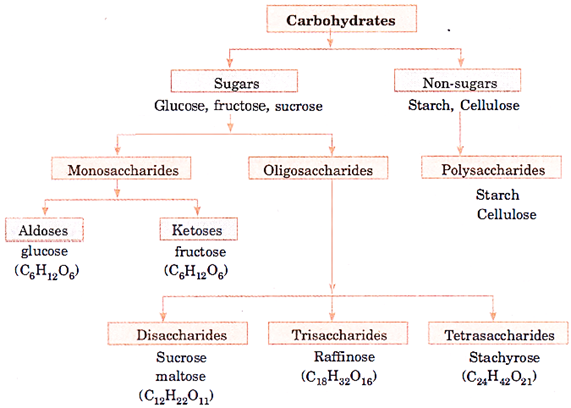
Classification of Monosaccharides
Monosaccharides are classified based on the number of carbon atoms and the functional group present in them.
Different types of monosaccharides are listed in the given table.
|
Carbon atoms |
General term |
Aldehyde |
Ketone |
|
3
|
Triose
|
Aldotriose
|
Ketotriose
|
|
4
|
Tetrose
|
Aldotetrose
|
Ketotetrose
|
|
5
|
Pentose
|
Aldopentose
|
Ketopentose
|
|
6
|
Hexose
|
Aldohexose
|
Ketohexose
|
|
7
|
Heptose
|
Aldoheptose
|
Ketoheptose
|
Glucose
Preparation of glucose
1. By boiling sucrose with dilute HCl or H2SO4 in alcoholic solution
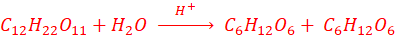
2. By boiling starch with dilute H2SO4, at 393 K, under pressure
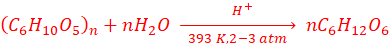
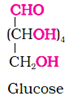
Glucose has been assigned the above structure based on the following evidences.
(i) Molecular formula − C6H12O6
(ii) Suggestion of straight chain
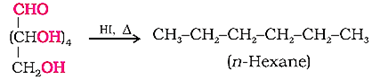
(iii) Confirmation of carbonyl (> C = O) group

(iv) Confirmation of the presence of carbonyl group as aldehydic group
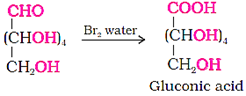
(v) Confirmation of the presence of five −OH groups
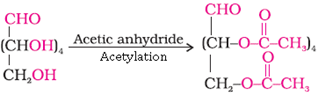
(vi) Indication of the presence of a primary alcohol
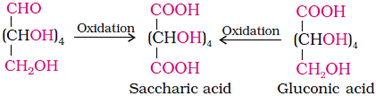
The correct configuration of glucose is given by
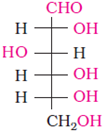
Glucose is correctly named as D (+) − Glucose
Cyclic Structure of Glucose
The following reactions of glucose cannot be explained by its open-chain structure.
Aldehydes give 2, 4-DNP test, Schiff’s test, and react with NaHSO3 to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
The penta-acetate of glucose does not react with hydroxylamine. This indicates that a free −CHO group is absent from glucose.
Glucose exists in two crystalline forms, α and β.
The α-form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the β-form (m.p. = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behaviour cannot be explained by the open-chain structure of glucose.
Hence it was proposed that Glucose exists in two cyclic forms, which exist in equilibrium with the open- chain structure.
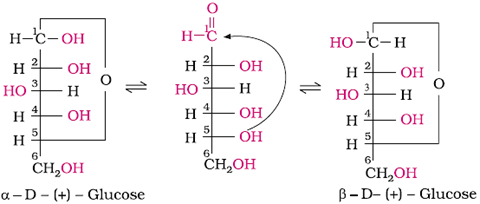
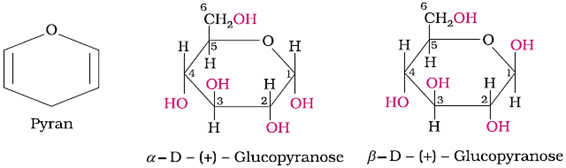
Representation of the cyclic structure of glucose by Haworth structure:
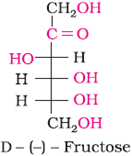
Structure of Fructose
Open-chain structure:
Cyclic structure:
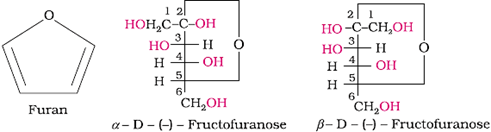
Representation of the structure of fructose by Haworth structures
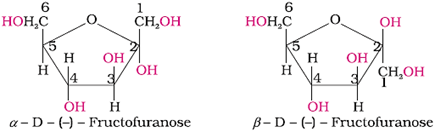
Disaccharides
Glycosidic linkage − Linkage between two monosaccharide units through oxygen atom
Sucrose (Non-reducing sugar)
Hydrolysis of sucrose:

Structure:
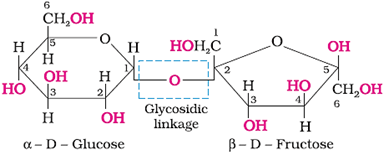
The product formed on the hydrolysis of sucrose is called invert sugar as the sign of rotation changes from dextro (+) of sucrose to laevo (−) of the product.
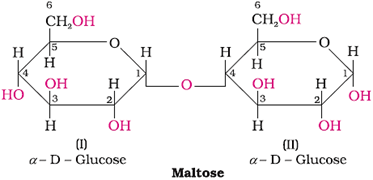
Maltose (Reducing sugar)
Structure:
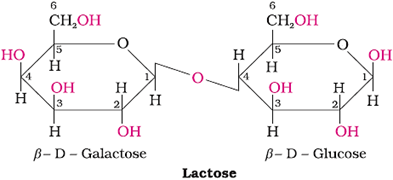
Lactose (Reducing sugar)
Commonly known as milk sugar
Polysaccharides
They mainly act as food storage or structural materials.
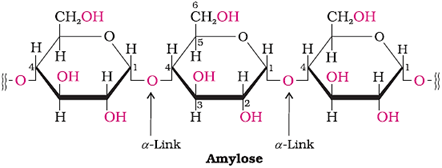
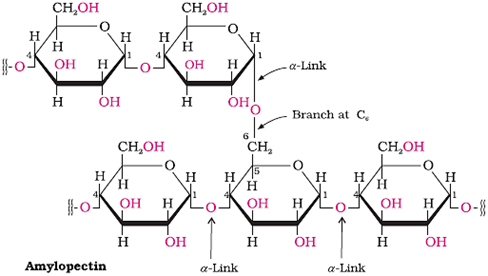
Starch
Main storage-polysaccharide of plants
Polymer of α-glucose; consists of two components − amylase and amylopectin
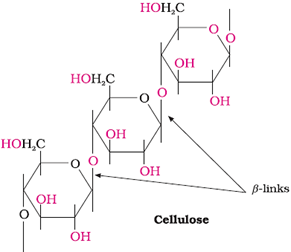
Cellulose
Predominant constituent of the cell wall of plant cells.
Straight-chain polysaccharide, composed of only β-D-Glucose
Glycogen
Storage-polysaccharide in animal body
Also known as animal starch because its structure is similar to amylopectin.
Proteins:
Proteins are polymers of α − amino acids.
Amino Acids
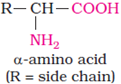
Some amino acids with their symbols are listed in the table given below.
|
Name |
Side
chain, R |
Three-letter
symbol |
One-letter
code |
|
1. Glycine |
H
|
Gly
|
G
|
|
2.
Alanine |
− CH3
|
Ala
|
A
|
|
3. Valine |
(H3C)2CH−
|
Val
|
V
|
|
4.
Leucine |
(H3C)2CH− CH2−
|
Leu
|
L
|
|
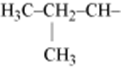
5. Isolecucine |
|
Ile
|
I
|
|
6.
Lysine |
H2N− (CH2)4 −
|
Lys
|
K
|
|
7. Glutamic acid |
HOOC − CH2 − CH2−
|
Glu
|
E
|
|
8.
Aspartic acid |
HOOC − CH2 −
|
Asp
|
D
|
|
9. Cysteine |
HS − CH2 −
|
Cys
|
C
|
|
10.
Methionine |
H3C− CH2 − CH2−
|
Met
|
M
|
|
11. Phenylalanine |
C6H5−CH2
−
|
Phe
|
F
|
|
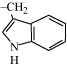
12. Tryptophan |
|
Trp
|
W
|
Classification of Amino Acids
Based on the relative number of amino and carboxyl groups, they are classified as acidic, basic and neutral.
Non-essential amino acids:
Amino acids that can be synthesised in the body
Example − Glycine, alanine, glutamic acid
Essential amino acids:
Amino acids that cannot be synthesised in the body, and must be obtained through diet
Example − Valine, leucine, isolecuine
Properties of Amino Acids
Colourless and crystalline solids
Exist as dipolar ions, known as zwitter ions, in aqueous solution.
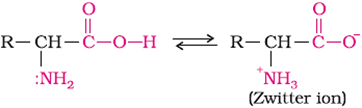
In zwitter form, amino acids show amphoteric behaviour.
All naturally occurring α-amino acids are optically active.
Structure of Proteins
Proteins are polymers of α-amino acids, joined to each other by peptide linkage or peptide bond.
Peptide linkage: Amide formed between −COOH group and −NH2 group of two amino acid molecules.
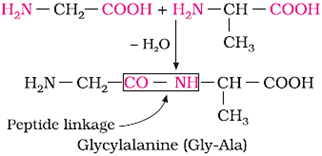
Dipeptide − Contains two amino acid molecules
Tripeptide − Contains three amino acid molecules
Polypeptide − Contains more than ten amino acid molecules
Based on the molecular shape, proteins are classified into two types −
Fibrous proteins
Globular proteins
Fibrous Proteins
In fibrous proteins, polypeptide chains run parallel and are held together by hydrogen and disulphide bonds.
Globular Proteins
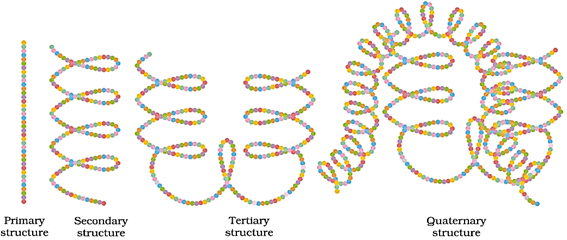
Polypeptide chains coil around, giving a spherical shape. Structures and shapes of proteins are studied at four different levels: primary, secondary, tertiary and quaternary.
Primary structure of proteins
Contains one or more polypeptide chains, and each chain has amino acids linked with each other in a specific sequence. This sequence of amino acids represents the primary structure of proteins.
Secondary structure of proteins
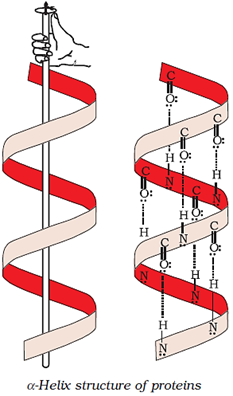
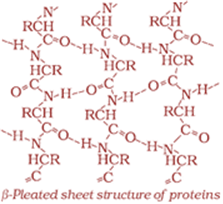
Shape in which a long polypeptide chain can exist; two types of secondary structures: α-helix, β-pleated sheet
α-helix structure of protein
β-pleated sheet structure
Tertiary structure of proteins
Overall folding of the polypeptide chains; results in fibrous and globular proteins; secondary and tertiary structures of proteins are stabilised by hydrogen bonds, disulphide linkages, van der Waals forces and electrostatic forces.
Quaternary structure of proteins
Spatial arrangement of subunits, each containing two or more polypeptide chains
The diagrammatic representations of the four structures of proteins are given below.
Denaturation of Proteins
Loss of biological activity of proteins due to the unfolding of globules and uncoiling of helix. Example − Coagulation of egg white on boiling, curdling of milk
Enzymes
Enzymes are biocatalysts.
Specific for a particular reaction and for a particular substrate
For example, maltase catalyses hydrolysis of maltose

The name of an enzyme ends with ‘−ase’.
They reduce the magnitude of activation energy
Vitamins
Organic compounds required in the diet in small amounts to maintain normal health, growth and nutrition
Classified into groups −
Water-soluble vitamins: Vitamin C, B-group vitamins (B1, B2, B6, B12)
Fat-soluble vitamins: Vitamins A, D, E and K
Some vitamins with their functions, sources and the diseases caused by their deficiency:
|
Vitamin |
Function |
Source |
Deficiency Diseases |
|
Vitamin A |
Maintenance of normal vision and healthy epithelial tissue |
Fish liver oil, carrots, butter and milk |
Xerophthalmia and night blindness |
|
Vitamin
B1 |
Synthesis of
ATP in the cell |
Yeast, milk,
green vegetables and cereals |
Beriberi |
|
Vitamin B2 |
Conversion of carbohydrates into glucose
1.
Protection of the cells and
DNA from free radicals |
Milk, egg-white, liver, kidney |
Cheilosis, digestive disorders and burning sensation of the skin |
|
Vitamin
B6 |
1.
Synthesis of antibodies and haemoglobin
2.
Maintenance of normal nerve function
3.
Breakdown of proteins
4.
Regulation of blood sugar |
Yeast, milk,
egg yolk, cereals and gram |
Convulsions |
|
Vitamin B12 |
Formation of RBC and maintenance of CNS |
Meat, fish, egg and curd |
Pernicious anaemia |
|
Vitamin
C |
1.
Maintenance of teeth and gums
2.
Repairing of tissues in the body
3.
Inhibition of histamine
4.
Improvement of body defence mechanism |
Citrus fruits, amla
and green leafy vegetables |
Scurvy |
|
Vitamin D |
Absorption of calcium required for the growth of bones |
Exposure to sunlight, fish and egg yolk |
Rickets and osteomalacia |
|
Vitamin
E |
1.
Improvement of the immune system
2.
Formation of RBC
3.
To prevent the blood clotting inside blood vessels |
Vegetable oils
like wheat germ oil, sunflower oil |
Increased fragility
of RBC and muscular weakness |
|
Vitamin K |
Required for normal blood clotting and synthesis of proteins found in
plasma |
Green leafy vegetables |
Delay of blood clotting |
Nucleic Acids
Two types: (i) Deoxyribonucleic acid (DNA) (ii) Ribonucleic acid (RNA)
Chemical composition of nucleic acids:
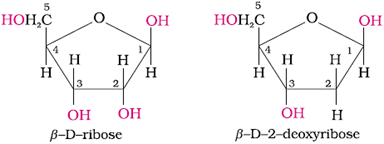
Nucleic acid contains a pentose sugar, phosphoric acid and a base (heterocyclic compound containing nitrogen).
In DNA, sugar is β-D-2-deoxyribose; in RNA, sugar is β-D-ribose
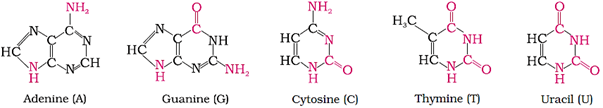
Bases in DNA: Adenine (A), guanine (G), cytosine (C) and thymine (T)
Bases in RNA: Adenine (A), guanine (G), cytosine (C) and uracil (U)
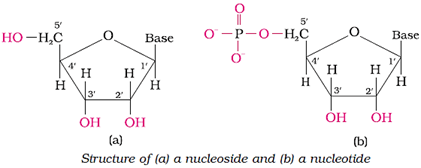
Structure of nucleic acids
Structure of a nucleoside and a nucleotide:
Formation of a di-nucleotide:
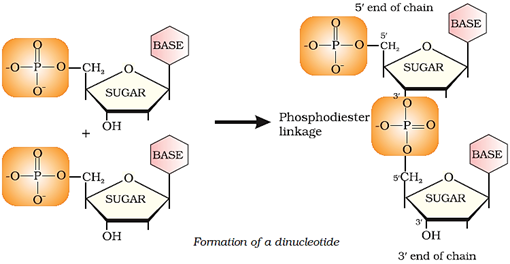
In secondary structure, the helices of DNA are double-stranded while those of RNA are single-stranded.
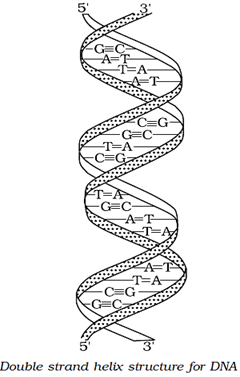
The two strands of DNA are complementary to each other.
Reason: H-bonds are formed between specific pairs of bases.
Double-strand helix structure of DNA:
Types of RNA:
Messenger RNA (m-RNA)
Ribosomal RNA (r-RNA)
Transfer RNA (t-RNA)
Functional differences between RNA and DNA:
|
RNA |
DNA |
|
|
1.
|
RNA is not responsible for heredity.
|
DNA is the chemical basis of heredity.
|
|
2.
|
Proteins are synthesised by RNA molecules in the cells.
|
DNA molecules do not synthesise proteins, but transfer coded messages for the synthesis of proteins in the cells.
|
Online Tuitions and Self-Study Courses for Grade 6 to 12 & JEE / NEET